
The consequences of ocean acidification
The pH value The pH value is a measure of the strength of acids and bases in a solution. It indicates how acidic or basic a liquid is. The pH scale ranges from 0 (very acidic) to 14 (very basic). The stronger an acid is the more easily it loses protons (H+), which determines the pH value. Practically expressed, the higher the proton concen-tration is, the more acidic a liquid is, and the lower its pH value is.
How climate change acidifies the oceans
Carbon dioxide is a determining factor for our climate and, as a greenhouse gas, it contributes considerably to the warming of the Earth’s atmosphere and thus also of the ocean. The global climate has changed drastically many times through the course of Earth history. These changes, in part, were associated with natural fluctuations in the atmospheric CO2 content, for example, during the transitions from ice ages to interglacial periods (the warmer phases within longer glacial epochs). The drastic increase in atmospheric CO2 concentrations by more than 30 per cent since the beginning of industrialization, by contrast, is of anthropogenic origin, i.e. caused by humans.
The largest CO2 sources are the burning of fossil fuels, including natural gas, oil, and coal, and changes in land usage: clearing of forests, draining of swamps, and expansion of agricultural areas. CO2 concentrations in the atmosphere have now reached levels near 390 ppm (parts per million). In pre-industrial times this value was only around 280 ppm. Now climate researchers estimate that the level will reach twice its present value by the end of this century. This increase will not only cause additional warming of the Earth. There is another effect associated with it that has only recently come to the attention of the public – acidification of the world ocean.
- There is a permanent exchange of gas between the air and the ocean. If the CO2 levels in the atmosphere increase, then the concentrations in the near-surface layers of the ocean increase accordingly. The dissolved carbon dioxide reacts to some extent to form carbonic acid. This reaction releases protons, which leads to acidification of the seawater. The pH values drop. It has been demonstrated that the pH value of seawater has in fact already fallen, parallel to the carbon dioxide increase in the atmosphere, by an average of 0.1 units. Depending on the future trend of carbon dioxide emissions, this value could fall by another 0.3 to 0.4 units by the end of this century. This may appear to be negligible, but in fact it is equivalent to an increased proton concentration of 100 to 150 per cent.
The effect of pH on the metabolism of marine organisms
The currently observed increase of CO2 concentrations in the oceans is, in terms of its magnitude and rate, unparalleled in the evolutionary history of the past 20 million years. It is therefore very uncertain to what extent the marine fauna can adapt to it over extended time periods. After all, the low pH values in seawater have an adverse effect on the formation of carbonate minerals, which is critical for many invertebrate marine animals with carbonate skeletons, such as mussels, corals or sea urchins.
Processes similar to the dissolution of CO2 in seawater also occur within the organic tissue of the affected organisms. CO2, as a gas, diffuses through cell membranes into the blood, or in some animals into the hemolymph, which is analogous to blood. The organism has to compensate for this disturbance of its natural acid-base balance, and some animals are better at this than others. Ultimately this ability depends on the genetically determined efficiency of various mechanisms of pH and ion regulation, which depends on the animal group and lifestyle. In spite of enhanced regulatory efforts by the organism to regulate them, acid-base parameters undergo permanent adjustment within tissues and body fluids. This, in turn, can have an adverse effect on the growth rate or reproductive capacity and, in the worst case, can even threaten the survival of a species in its habitat.
- The pH value of body fluids affects biochemical reactions within an organism. All living organisms therefore strive to maintain pH fluctuations within a tolerable range. In order to compensate for an increase in acidity due to CO2, an organism has two possibilities: It must either increase its expulsion of excessive protons or take up additional buffering substances, such as bicarbonate ions, which bind protons. For the necessary ion regulation processes, most marine animals employ specially developed epithelia that line body cavities, blood vessels, or the gills and intestine.
- 2.6 > By studying ice cores scientists want to discover which organisms live in the ice. Air bubbles in Antarctic ice cores also provide clues to the presence of trace gases in the former atmosphere, and to past climate. The ice cores are drilled using powerful tools. For more detailed study they are analysed in the laboratory. When ice crystals are observed under a special polarized light, their finestructure reveals shimmering colours.

- The ion transport systems used to regulate acid-base balance are not equally effective in all marine animal groups. Marine organisms are apparently highly tolerant of CO2 when they can accumulate large amounts of bicarbonate ions, which stabilize the pH value. These organisms are usually also able to very effectively excrete protons. Mobile and active species such as fish, certain crustaceans, and cephalopods – cuttlefish, for instance – are therefore especially CO2-tolerant. The metabolic rates of these animals can strongly fluctuate and reach very high levels during exercise (hunting & escape behaviour). The oxygen-consumption rate (a measure of metabolic rate) of these active animal groups can reach levels that are orders of magnitude above those of sea urchins, starfish or mussels. Because large amounts of CO2 and protons accumulate during excessive muscle activity, active animals often possess an efficient system for proton excretion and acid-base regulation. Consequently, these animals can better compensate for disruptions in their acid-base budgets caused by acidification of the water.
Benthic invertebrates (bottom-dwelling animals without a vertebral column) with limited ability to move great distances, such as mussels, starfish or sea urchins, often cannot accumulate large amounts of bicarbonate in their body fluids to compensate for acidification and the excess protons. Long-term experiments show that some of these species grow more slowly under acidic conditions. One reason for the reduced growth could be a natural protective mechanism of invertebrate animal species: In stress situations such as falling dry during low tide, these organisms reduce their metabolic rates. Under normal conditions this is a very effective protection strategy that insures survival during short-term stress situations. But when they are exposed to long-term CO2 stress, this protective mechanism could become a disadvantage for the sessile animals. With the long-term increase in carbon dioxide levels in seawater, the energy-saving behaviour and the suppression of metabolism inevitably leads to limited growth, lower levels of activity, and thus a reduced ability to compete within the ecosystem.
- 2.9 > Diatoms like this Arachnoidiscus are an important nutrient basis for higher organisms. It is still uncertain how severely they will be affected by acidification of the oceans.

- However, the sensitivity of a species’ reaction to CO2 stressor and acidification cannot be defined alone by the simple formula: good acid-base regulation = high CO2 tolerance. There are scientific studies that suggest this is not the case. For example, one study investigated the ability of a species of brittlestar (echinodermata), an invertebrate that mainly lives in the sediment, to regenerate severed arms. Surprisingly, animals from more acidic seawater not only re-grew longer arms, but their calcareous skeletons also contained a greater amount of calcium carbonate. The price for this, however, was reduced muscle growth. So in spite of the apparent positive indications at first glance, this species is obviously adversely affected by ocean acidification because they can only efficiently feed and supply their burrows in the sediment with oxygenated seawater if they have fully functioning arms.
- Even fish can be impaired. Many adult animals are relatively CO2 tolerant. Early developmental stages, however, obviously react very sensitively to the CO2 stressor. A strong impairment of the sense of smell in seawater with low pH values was observed in the larval clownfish. These animals are normally able to orientate themselves by a specific odour signal and, after their larval phase, which they spend free-swimming in the water column, to find their final permanent habitat on coral reefs. In the experiment, fish larvae that were raised in seawater with a pH value lowered by about 0.3 units, reacted significantly less to the otherwise very stimulating odour of sea anemones with which they live in symbiosis on reefs. If behavioural changes caused by CO2 occur during a critical phase of the life cycle, they can, of course, have a strong impact on the reproductive success of a species.
It is not yet known to what extent other marine organisms are impacted by these kinds of effects of ocean acidification. Other studies on embryonic and juvenile stages of various species have shown, however, that the early developmental stages of an organism generally respond more sensitively to CO2 stress than the adult animals do.
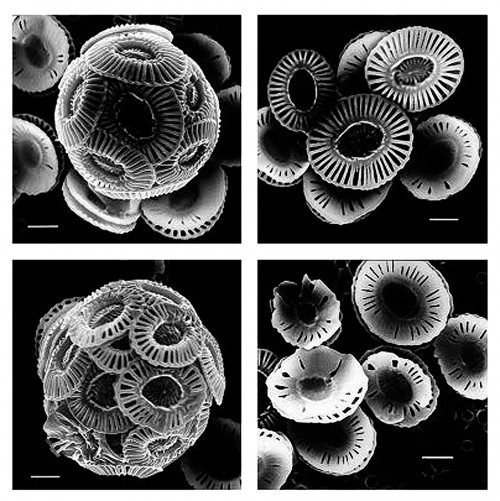
- 2.10 > These electron micrographs clearly illustrate that increased CO2 concentrations in the water can disturb and lead to malformation in calcareous marine organisms, such as the coccolithophorid Emiliana huxleyi shown here. The upper pictures reflect CO2 concentrations in the water of 300 ppm, which is slightly above the pre-industrial average CO2 level for seawater. The bottom photographs reflect a CO2 content of 780 to 850 ppm. For size comparison, the bars represent a length of one micrometre.
 2.11 > The clownfish (Amphiprion percula) normally does not react sensitively to increased CO2 concentrations in the water. But in the larvae the sense of smell is impaired.
2.11 > The clownfish (Amphiprion percula) normally does not react sensitively to increased CO2 concentrations in the water. But in the larvae the sense of smell is impaired.Threat to the nutrition base in the oceans – phytoplankton and acidification
The the entire food chain in the ocean is represented by the microscopic organisms of the marine phytoplankton. These include diatoms (siliceous algae), coccolithophores (calcareous algae), and the cyanobacteria (formerly called blue algae), which, because of their photosynthetic activity, are responsible for around half of the global primary productivity. Because phytoplankton requires light for these processes, it lives exclusively in surface ocean waters. It is therefore directly affected by ocean acidification. In the future, however, due to global warming, other influencing variables such as temperature, light or nutrient availability will also change due to global warming. These changes will also determine the productivity of autotrophic organisms, primarily bacteria or algae, which produce biomass purely by photosynthesis or the incorporation of chemical compounds. It is therefore very difficult to predict which groups of organisms will profit from the changing environmental conditions and which will turn out to be the losers. Ocean acidification is of course not the only consequence of increased CO2. This gas is, above all, the elixir of life for plants, which take up CO2 from the air or seawater and produce biomass. Except for the acidification problem, increasing CO2 levels in seawater should therefore favour the growth of those species whose photosynthetic processes were formerly limited by carbon dioxide. For example, a strong increase in photosynthesis rates was reported for cyanobacteria under higher CO2 concentrations. This is also true for certain coccolithophores such as Emiliania huxleyi. But even for Emiliania the initially beneficial rising CO2 levels could become fatal. Emiliania species possess a calcareous shell comprised of numerous individual plates. There is now evidence that the formation of these plates is impaired by lower pH values. In contrast, shell formation by diatoms, as well as their photosynthetic activity, seems to be hardly affected by carbon dioxide. For diatoms also, however, shifts in species composition have been reported under conditions of increased CO2 concentration.
Challenge for the future: Understanding acidification
In order to develop a comprehensive understanding of the impacts of ocean acidification on life in the sea, we have to learn how and why CO2 affects various physiological processes in marine organisms. The ultimate critical challenge is how the combination of individual processes determines the overall CO2 tolerance of the organisms. So far, investigations have mostly been limited to short-term studies. To find out how and whether an organism can grow, remain active and reproduce successfully in a more acidified ocean, long term (months) and multiple-generation studies are neccessary.
- The final, and most difficult step, thus is to integrate the knowledge gained from species or groups at the ecosystem level. Because of the diverse interactions among species within ecosystems, it is infinitely more difficult to predict the behaviour of such a complex system under ocean acidification.
- In addition, investigations are increasingly being focused on marine habitats that are naturally characterized by higher CO2 concentrations in the seawater. Close to the Italian coast around the island of Ischia, for instance, CO2 is released from the sea floor due to volcanic activity, leading to acidification of the water. This means that there are coastal areas directly adjacent to one another with normal (8.1 to 8.2) and significantly lowered pH values (minimum 7.4). If we compare the animal and plant communities of these respective areas, clear differences can be observed: In the acidic areas rock corals are completely absent, the number of specimens of various sea urchin and snail species is low, as is the number of calcareous red algae. These acidic areas of the sea are mainly dominated by seagrass meadows and various non-calcareous algal species.
- 2.12 > Low pH values in the waters around Ischia cause corrosion of the shells of calcareous animals such as the snail Osilinus turbinata. The left picture shows an intact spotted shell at normal pH values of 8.2. The shell on the right, exposed to pH values of 7.3, shows clear signs of corrosion. The scale bars are equal to one centimetre.
 The further development of such ecosystem-based studies is a great challenge for the future. Such investigations are prerequisite to a broader understanding of future trends in the ocean. In addition, deep-sea ecosystems, which could be directly affected by the possible impacts of future CO2 disposal under the sea floor, also have to be considered.
In addition, answers have to be found to the question of how climate change affects reproduction in various organisms in the marine environment. Up to now there have been only a few exemplary studies carried out and current science is still far from a complete understanding. Whether and how different species react to chemical changes in the ocean, whether they suffer from stress or not is, for the most part, still unknown. There is an enormous need for further research in this area.
The further development of such ecosystem-based studies is a great challenge for the future. Such investigations are prerequisite to a broader understanding of future trends in the ocean. In addition, deep-sea ecosystems, which could be directly affected by the possible impacts of future CO2 disposal under the sea floor, also have to be considered.
In addition, answers have to be found to the question of how climate change affects reproduction in various organisms in the marine environment. Up to now there have been only a few exemplary studies carried out and current science is still far from a complete understanding. Whether and how different species react to chemical changes in the ocean, whether they suffer from stress or not is, for the most part, still unknown. There is an enormous need for further research in this area. 