Searching for the causes of disease
-
Model marine organisms Cnidarians and sponges are among the oldest life forms on Earth. They have been living in the oceans for hundreds of millions of years. In spite of their simple body structure they possess an amazing number of genes. These control metabolic processes that have been lost to some extent by higher organisms during the course of evolution. For this reason cnidarians and sponges can be viewed as a kind of prototype for all animals, and represent ideal models to study the basic principles of life.
How does an organism protect itself from pathogens?
The first line of defence against potential pathogens in humans, other vertebrates, and invertebrates such as sponges, is natural immunity. Even infants have an innate immune system, although they have hardly been exposed to any pathogens. This ancient phylogenetic defence mechanism consists of scavenger cells that destroy germs (phagocytosis), metabolic processes that attack and dissolve foreign proteins, and the production of antimicrobial peptides. These peptides are found in animals, plants and microorganisms. They are produced by certain body tissues such as the intestine, skin and lungs, and provide protection against infection. The human immune defence system – or at least part of it – is very old and is related to the lower-order organisms. These organisms include sponges and cnidarians (corals, jellyfish, sea anemones and freshwater polyps), which have lived in the sea for hundreds of millions of years in constant contact with bacteria and viruses. For this reason it is quite possible that they can teach scientists how an efficient defence system develops and how it can be mended in the event of disease.
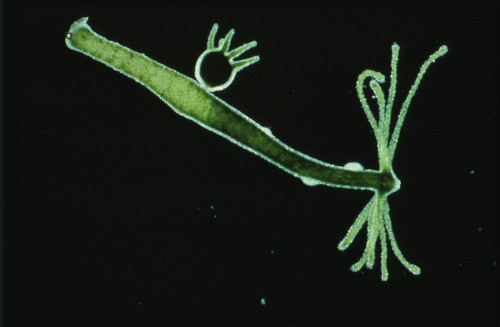
Cnidarians, among the most primordial of sea-dwellers, seem ideally suited to the study of how an organism keeps bacteria and other pathogens at bay. They are relatively simple organisms, but nonetheless numerous complex metabolic processes take place within and between their body cells. At first glance cnidarians appear to be vulnerable and defenceless against pathogens because they have neither immune cells to destroy pathogenic intruders nor a lymphatic system to circulate defence cells through the body. They also lack a solid protective covering, having only an outer layer of cells, the epithelium. They have nonetheless managed to survive for millions of years. This makes them extremely interesting subjects of study.
- Researchers are trying to find out how their tissue interacts with microbes, and how the metabolic processes in their outer skin fend off enemies. They have successfully bred genetically-modified cnidarians in which the antibacterial defence molecules are visible. This enables them to examine the living creature to see both where the antibodies are released and where they are deployed. It seems amazing that such weak and insignificant little creatures can survive in an environment that is literally teeming with potential pathogens, despite their lack of an immune system and patrolling immune cells. As we know, the exterior surface of many marine creatures, such as sponges, is permanently colonized by bacteria. And furthermore, a litre of seawater can contain up to 2 trillion bacteria and an even greater number of viruses. These microorganisms include many potential pathogens. Despite all this the creatures survive. If we wish to gain greater insight into how the body interacts with its environment, and to explore the principles of evolution, ancient marine organisms seem to be the ideal models. Thanks to new analytical capabilities, cnidarians play an interesting role in trying to understand the evolution of immune reactions, identifying the genes involved and explaining the universal mechanisms of animalmicroorganism interaction.
- 9.11 > Sea anemones belong to the species-rich phylum Cnidaria. They are related to corals and jellyfish.

The body and its bacteria – a finely-tuned instrument
Higher life forms and bacteria are in constant contact with each other. Bacteria act either as disease-causing pathogens or as symbionts that take over some vital functions. For example, the intestine is colonized by a complex and dynamic community of microorganisms that support a range of metabolic functions. The intestine is gradually colonized by bacteria from birth onwards, continuing through the early stages of life until each individual ultimately develops his or her own specific intestinal flora.
Questions that largely remain unexplained include how the intestinal epithelium (the outer cell layer) interacts with the microorganisms, how the body differentiates between useful bacteria and potential pathogens, and how the bacteria influence the metabolic processes and efficiency of the human intestinal epithelium. It is possible that studies of Cnidaria could help here. Their epithelium, or outer body surface, is also colonized by a complex and dynamic community of microorganisms. Tests on the freshwater polyp Hydra have shown that individuals from different Hydra species differ greatly in the composition of their microfauna.
- Having said that, however, when the bacterial composition of individuals kept under controlled laboratory conditions for many years, are compared to individuals of the same species taken directly from their natural habitat, the results are strikingly similar. This means that the colonizing bacteria remain faithful to the Hydra individuals for long periods. They are constant inhabitants of the epithelium.
These findings suggest that a rigorous selection process is at work on the epithelium. It appears that under specific conditions, certain bacterial communities that are ideally suited to the habitat become established on the epithelial tissue, remaining constant for a long time. These observations as well suggest that the epithelium actively shapes the composition of the microbial community. In case the bacterial growth on mammals or invertebrates is removed, the animals usually fall ill. The metabolic system is disrupted and the immune system weakened. Disorders of the alimentary canal are extremely severe. The animals can no longer defend themselves against infections caused by pathogenic bacteria and viruses.
- We are also aware that certain genetic defects in the human immune system can disrupt the collaboration between the epithelium and its colonizing microbes. People affected are usually prone to inflammatory diseases of the barrier organs – the parts of the body that are open to the outside world, such as the skin and lungs. Although we have no definite immunobiological explanation for the effect of the microbes, it is clear that symbiotic bacteria actively contribute to the critical balance between health and disease.
- Bacteria are therefore essential to a large number of organisms. For instance, during its juvenile stage the bioluminescent squid Euprymna scolopes develops light organs on the surface of its skin. Like a firefly, the squid is therefore capable of generating light pulses by means of a biochemical reaction. However, the light organs cannot grow without a certain component being contributed by Vibrio fischeri, a symbiotic bacterium present in the epithelium of the squid. It is therefore clear that both the physical development and the immune system of verte brates and invertebrates are significantly affected by their colonizing microorganisms. However, little is understood about how bacteria influence the immune functions and the mechanisms that control the complex interactions between the microbial communities and the animals. Neither do we know how the metabolism of the epithelium impacts the composition of the symbiotic bacterial community. Initial experiments on the polyp Hydra showed that changes to the cells do in fact alter the bacterial flora. When a certain type of cell was removed from the tissue, the bacterial composition on Hydra’s body surface changed conspicuously. The marked decrease of normally predominant proteobacteria was accompanied by a similar increase in the formerly rare bacteria of the bacteriodetes group. There certainly appears to be a direct interaction between the host epithelium and the microbes.
A new understanding of human disease
A large number of modern human diseases stem from dysfunctions of the boundary between the body and the outside world. These include chronic inflammatory diseases of the barrier organs, those organs that are in contact with the external environment – the skin, the lungs and the intestine, which is fed from outside sources. Examples are bronchial asthma (lungs), psoriasis and neurodermatitis (skin) as well as Crohn’s disease and ulcerative colitis, chronic inflammatory bowel diseases (intestine).
Interestingly, these conditions are entirely unknown in animals. Systematic genetic tests have shown that many of them are triggered by so-called risk genes, which are ancient in an evolutionary sense. It is ironic that such complaints have proliferated in recent years, especially in the industrial nations. All diseases have one thing in common, that the human immune system breaks down at the barriers to the environment, and attacks its own body structures. New technologies have enabled us to trace individual abnormal elements on the molecular roadmap for disease. These individual components should now be combined to create an overall model for understanding the mechanisms underlying malfunctions of the immune system.
- Current research indicates that immune system malfunction involves a large number of genes that control the evolutionarily old forms of immunological engagement with the environment, such as the surrounding microbiota.
One question is how, during the course of evolution, genetic variability could occur in those genes that determine the characteristics of the barriers. How do erratic food conditions or different microbiota in the water change the genetic variability of the barriers? How do such changes influence the evolutionary fitness of organisms or, in other words, the likelihood of genes surviving and reproducing? Understanding the processes on the surface of marine animals may help us to comprehend how diseases of the barrier organs occur in humans. Once we have unravelled the evolution and function of the barrier organs, we may find new strategies to treat or even prevent these diseases. Over past decades selected model organisms such as the mouse and the fruit fly Drosophila melanogaster have taught us a lot about recognizing and fighting the triggers of disease. But even today the question of why the outer skin of all organisms is colonized by microbes and how these microbes interact with the host remains a mystery.