
Over-fertilization of the seas
Rivers – the lifeblood of coastal waters
Coastal waters are among the most productive regions of the oceans. The greatest numbers of fish, shellfish and seafood in general are caught here. The high productivity is a result of nutrients that are transported by rivers from the land into the sea. These mainly comprise phosphate and nitrogen compounds, which plants require for growth. Phytoplankton in the ocean, microscopically small algae in particular, also utilizes these substances. Because of the high availability of nutrients, phytoplankton grows exceptionally well in coastal regions. It is consumed by zooplankton, small crustaceans, fish larvae, and other creatures, and thus forms the base of the food web in the ocean.
The high productivity of coastal waters also makes them increasingly attractive areas for aquaculture. The output of the aquaculture industry increased worldwide by a factor of fifteen between 1970 and 2005. But rivers are not the only source of nutrients for coastal areas. On the west coast of Africa, for instance, ocean currents from greater depths bring nutrient-rich water up to the surface, where light can penetrate. In these upwelling regions, the nutrients also promote a rich growth of algae, increase productivity through the entire food web, and ultimately produce a greater yield for fisheries. A natural level of nutrients is therefore a positive factor and is essential for marine organisms in the coastal waters.
- 4.1 > Eutrophication stimulates the growth of algae, which are sometimes pounded to foam in the surf, as seen here on the German North Sea coast.

Too much of a good thing
In many densely populated regions of the Earth, however, excessive amounts of nutrients are finding their way into the coastal waters. A large proportion of these nutrients come from the intensive agricultural application of chemical fertilizers, which are washed by rain into the rivers.
Between 1970 and 2005 the amount of nitrogen fertilizer alone, applied globally, increased by almost a factor of three. Nitrogen and phosphate compounds are also transported to the sea by untreated wastewater, and via the atmosphere from the burning of fossil fuels. The production and decay of organic material are unnaturally intensified by the huge amounts of nutrients in coastal waters. Scientists call this process eutrophication. The availability of nutrients is so great that the phytoplankton population grows beyond normal levels, producing a classic algal bloom. In the North Sea and in the Wadden Sea, massive algal occurrences are occasionally whipped into a foam by the surf. These sometimes form piles up to a metre high, resembling giant meringues. A serious threat is presented by the propagation of toxic algae.
- 4.2 > Over-fertilization of the seas usually first becomes apparent with the appearance of copious amounts of green algae. Prior to the start of the Olympic sailing competition in Qingdao in 2008, the algae had to be removed from the water surface by hand.

 4.3 > When conditions are favourable for phytoplankton growth, algal blooms occur in the oceans, as here in the Baltic Sea. Through the massive reproduction of cyanobacteria, formerly called blue-green algae, the water in these areas turns green. Such phenomena are completely natural, but because of over-fertilization these blooms are occurring with unusually high frequency today.
4.3 > When conditions are favourable for phytoplankton growth, algal blooms occur in the oceans, as here in the Baltic Sea. Through the massive reproduction of cyanobacteria, formerly called blue-green algae, the water in these areas turns green. Such phenomena are completely natural, but because of over-fertilization these blooms are occurring with unusually high frequency today.- These are poisonous to various organisms in the sea, such as fish and clams and if they enter the food chain, they may also be ingested by humans. Numerous cases have been reported of people dying after eating poisoned shellfish. Scientists have also verified the deaths of marine mammals from algal toxins that they ingested with their food. These toxic algal blooms occur along the coast of Texas, for example. Because they discolour the water they are commonly called “red tides” or “brown tides”.
The blooms of non-toxic algae can also create problems when the algae die. The dead algae sink to the bottom where they are broken down by microorganisms through a process that depletes oxygen in the seawater. Low oxygen concentrations in the water can lead to large-scale mortality of fish and crustaceans. When the oxygen levels begin to drop, the animals that can actively move, such as fish and crabs, leave the area first. Within the sea floor, the population of animals that require a healthy oxygen supply diminishes at the same time. If the oxygen concentration continues to drop, then most of the other species living in the sea floor also disappear. Only a few species that can tolerate low oxygen levels remain. If the bottom water finally becomes completely depleted of oxygen, even these organisms will die off.
But eutrophication also causes blooms of other organisms besides phytoplankton. It has a significant effect on larger plants, and can often change entire coastal ecosystems. One example of this was the formation of a vast carpet of green algae on the Chinese coast at Qingdao in 2008, which disrupted the Olympic sailing competition. In other cases, eutrophication leads to the disappearance of seagrass beds (Chapter 5) or to changes in the species composition in certain habitats. In short, eutrophication is an illustration of how changes onshore can impact the ocean, because the oceans are connected to the land masses by rivers and the atmosphere. To counteract the negative effects of eutrophication, serious efforts are being made to reduce the input of phosphate and nitrogen compounds into coastal waters.
Reversing the trend
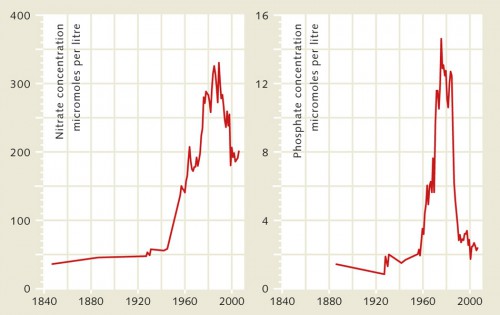
The Rhine River and North Sea present a good example illustrating how the input of nutrients by rivers into the ocean has evolved through time in European regions, because extensive data are available for both of these water bodies. The first observations were made as early as the mid 19th century. Water samples from the Rhine near the border of Germany and Holland were taken and analysed over several decades. Near the border town of Lobith, researchers documented a strong increase in phosphate and nitrate concentrations from the mid-20th century. Appropriate measures were taken that have succeeded in consistently reducing the concentrations since the mid-1980s.
The causes of the increase included a growing input from agriculture and industry as well as the discharge of untreated urban sewage. Laundry detergent with phosphate additives to decalcify the wash water was a significant source of phosphates. As early as the 1970s, a ban on this type of detergent had already begun to reduce the phosphate concentrations in the Rhine. Then, in the 1980s, the nitrogen levels in the river also began to drop. This can be attributed in part to improved fertilizing methods in agriculture that resulted in lesser amounts of nutrients being washed from the fields. Another reason is the improved treatment of industrial and domestic wastewater. In 1987, environmental ministers from the North Sea countries finally agreed to a goal of halving the amounts of phosphate and nitrogen transported by rivers. For phosphates this goal was reached quickly. For the nitrogen compounds it took almost 25 years. Despite decreasing phosphate and nitrogen concentrations in the water, however, the Rhine River still carries large amounts of nutrients to the North Sea, because it flows through a highly developed and intensively used agrarian region. The present nitrate loads are still higher than in the pre-industrial age 150 years ago. Similar situations exist in other European river regions and in the USA.
- 4.4 > Eutrophication in coastal waters is primarily caused by an abundance of nitrates (nitrogen compounds) and phosphates that are washed into the ocean by large rivers. For example, since the middle of last century the concentration of nutrients in the Rhine River near the border town of Lobith has increased enormously. This is largely due to the intensive use of chemical fertilizers in agriculture and inadequate wastewater treatment. Counteractive measures such as a ban on phosphate detergents and improved fertilizing techniques have been successful in significantly reducing the input since the 1980s. But in many other coastal regions of the world the nutrient concentrations continue to increase.
- In some parts of Europe, political decisions have thus led to a reversal of the trends and a reduction of nutrient input into the oceans. But the opposite trend can be observed globally. Computer models indicate that the use of fertilizer is increasing in many regions due to population growth and the intensification of agriculture. Accordingly, in many coastal regions, the amounts of phosphate and nitrogen being washed into the sea by the rivers are increasing. Particularly in Southeast Asia, rivers are carrying more and more nutrients to the sea, and experts expect this trend to continue.
Extra Info The Mississippi River and the Gulf of Mexico dead zone

A global problem
The effects of eutrophication have been coming to light since the 1960s. Researchers have noted more abundant algal blooms, oxygen-poor zones in coastal regions, and changes in coastal ecosystems. The causes of eutrophication have been thoroughly analysed in numerous studies, and there is certainly a direct connection between environmental changes and nutrient input. But for a long time researchers were in disagreement as to how the phosphates and nitrates interact as nutrients. Some experts accepted that the “law of the minimum”, formulated by the agronomist Carl Sprengel in 1828, was valid for algal growth. According to this theory, a plant requires several nutrients in order to thrive. If one nutrient is missing, then it cannot grow. This means that the growth of plants would always be limited by the one substance that is not available in sufficient quantity. This would suggest that it is sufficient to remove one nutrient, either phosphate or nitrogen, from the wastewater and rivers in order to stop the growth of algae. This would also significantly reduce the costs of water treatment.
This assumption, however, now appears to be too simplistic. Continuing experiments and observations show that multiple factors acting in concert are often responsible for limiting plant growth. Experts call this phenomenon co-limitation. Eutrophication can only be combated successfully if both phosphate and nitrogen are reduced. However, this is fraught with difficulty, primarily because nitrogen released by agricultural activity is not easily contained. This is also true of nitrogen released into the atmosphere by the burning of natural gas, oil or coal. Eutrophication is therefore likely to continue to occur in coastal waters in the future.
- One example of a strongly eutrophic area is the German Bight. In the 1980s the oxygen concentration in its deep waters dropped to alarming levels. At the same
time an increase in primary productivity in the form of enhanced algal growth was observed in the Wadden Sea. Seagrass, a plant that is the foundation for a unique habitat in the North Sea and Wadden Sea, disappeared. It was displaced by an excessive proliferation of green algae. All over the world, bays with limited water exchange are affected by eutrophication because nutrients are not effectively dispersed. These include Tokyo Bay, Long Island Sound in the USA, the Baltic Sea, and several of the fjords in Norway.
Eutrophication with an excessive growth of phytoplankton has also been observed in some areas in the Mediterranean Sea, such as the north-eastern Adriatic Sea or the bay at Athens. The Gulf of Mexico is a special case: here the Mississippi River discharges such a large volume of nutrients that an extensive low-oxygen area has formed along the coast.
Any chance of recovery?
Through systematic measures such as the Water Framework Directive of 2000, or the Marine Strategy Framework DirectiveMarine Strategy Framework Directiveadopted in 2008, the European Union is striving to improve water quality in the European coastal waters. Key parameters for evaluating water quality are sufficient oxygen content, low nutrient levels, and the presence of certain algal species and bottom dwellers. Wherever possible the previously eutrophic waters should be restored to their natural condition, or at least to an only slightly impacted state. Improved monitoring for ongoing assessment should also be carried out in order to identify changes and their causes.Further information on this topic is available here:
- Due to world population growth, eutrophication will continue to be a problem for decades to come. There is presently little hope of a worldwide reduction in the amounts of nutrients being discharged into coastal waters. A true dilemma exists: humankind has a vital need for agriculture and the production of grain, but this results in vast amounts of fertilizers ending up in the rivers and oceans. Often costly abatement measures are therefore required to achieve a balance between the nutrient input from agriculture and the negative impact on the ecosystem. One particular problem is that it is impossible to completely restore a coastal ecosystem affected by eutrophication to its original state. Eutrophication is not fully reversible. Studies in several European coastal systems indicate that a long period of eutrophication produces lasting changes in the ecosystem that cannot simply be reversed by reducing the nutrient input. Nonetheless, the example of the Wadden Sea clearly illustrates that practical measures can be effective in decreasing the amount of nutrients and creating a general improvement in the marine environment. In the northern Wadden Sea, for instance, there are indications that the seagrass beds have recovered and are expanding again as a result of the reduction of nutrients and algal blooms.
