
From plankton to hydrate
The discovery of a new resource
Methane is a commonly occurring molecule in wide-spread use: it is the principal combustible component of natural gas. Depending on its quality, natural gas contains 75 to 99 per cent methane. Additional components may include the gases ethane, propane or hydrogen sulphide. At room temperature and normal atmospheric pressure at the Earth’s surface, methane exists as a gas. At lower temperatures and higher pressures, however, it can, in the presence of water, form an ice-like solid called methane hydrate. In the hydrate, methane is compressed to a density of about 160 times that of nat-ural gas. This means that one cubic metre of hydrate contains about 160 cubic metres of gas. So with breakdown of the hydrate a huge amount of methane gas is released.
We have known about methane hydrate since the 1930s. At that time natural gas providers complained that their gas lines and valves would freeze up in cold weather. What was disconcerting was that sometimes they froze at temperatures above the freezing point of water. Clearly the blockage could not have been caused by normal water ice. Researchers discovered that the ice-like deposits were a substance composed of methane and water. Additives were subsequently added to the gas to prevent the undesirable formation of methane hydrates.
Initially methane hydrate was believed to be a phenomenon limited to industrial plants. But in the 1960s Russian scientists created a minor sensation when they unintentionally retrieved chunks of methane hydrate while drilling into the Earth’s surface.
- They thus provided solid evidence that methane hydrate could occur naturally. Soon thereafter U.S. researchers verified the presence of methane hydrate in the permafrost of Alaska. This led them to the assumption that methane hydrates could occur commonly in nature, so they began to search for it globally, including beneath the oceans. The first large occurrences were discovered in 1971 on the floor of the Black Sea, and in the early 1980s off the coast of Alaska. Today it is known that methane hydrate occurs in all oceans, primarily on the continental margins.
It is estimated that around ten times more methane is stored in hydrates below the sea floor than is present in all other conventional natural gas deposits. Methane hydrate is thus seen as a very promising fossil energy source for the future. Exploration for methane hydrate deposits in the seas has consequently intensified over the past 10 years. Particular interest has been shown by countries like Japan, South Korea and Taiwan. They have almost no fossil energy reserves of their own and therefore depend on the import of large quantities of gas, coal and oil. With methane hydrates from their own territorial waters they could significantly decrease their dependence on imports and their exposure to energy prices, which have recently risen steeply.
First methane, then hydrate
Methane hydrates develop naturally only in areas where sufficient methane is present. This gas can de-velop beneath the sea floor in two different ways:- Biogenic methane is formed in the sea floor by the microbial breakdown of biomass. The biomass consists of dead planktonic organisms such as micro-algae or krill that sink through the water column to the sea floor and build thick sediment packages over time. Methane-producing microorganisms break down the biomass into methane and carbon dioxide. This process is known as methanogenesis. Scientists estimate that 80 to 90 per cent of the methane stored in hydrates worldwide was produced biogenically by methanogenesis. The methanogenic bacteria are found at depths of around 10 metres to 3 kilometres in the sediment. Above this depth of 10 metres other microorganisms are active that do not produce methane. Microorganisms that require oxygen live directly on the sea floor and within the upper centimetres of sediment. These “aerobic”, or oxygen-feeding microorganisms, break down a large proportion of the sinking biomass. In the nearly oxygen-free sediments immediately below, on the other hand, microorganisms are active that require the sulphate radical for their metabolism, which is present in large amounts in these sediment layers. These organisms, called sulphate reducers, also consume biomass without producing methane. Only in the environment below 10 metres, lacking in both oxygen and sulphate, can the methanogenic microorganisms flourish.
- Thermogenic methane is generated chemically in the much deeper layers of the Earth’s crust without the activity of microorganisms. It is formed in a similar way as oil and natural gas. At depths of several kilometres, under high pressures and temperatures above 100 degrees Celsius, the remains of biomass millions of years old in hard sedimentary rocks are transformed into methane. This is achieved by purely chemical processes driven by heat. The thermogenic methane can then rise through fissures in the rocks up to the layers where pressure and temperature conditions allow the formation of hydrates.
- 3.3 > Gas hydrates occur where abundant biomass sinks to the bottom in areas of low temperature and high pressure – particularly on continental slopes. The higher the water temperature is, the greater the depths and pressures necessary for the formation of hydrates. At very great depths, due to the Earth’s geothermal energy, the temperature within the sea floor is too high for the formation of methane hydrate.

- Thus the requirements for the formation of methane hydrates are the right temperature, the right pressure, and a sufficiently high methane concentration. These conditions are commonly found in areas near the coasts, particularly on the continental slopes at water depths below 500 metres. Most coastal areas are rich in nutrients, which are transported by rivers to the sea. Vast numbers of planktonic organisms thrive here, in turn providing food for higher animals. Coastal areas are therefore immensely productive and the amount of dead biomass that settles to the sea floor and is deposited as sediments is large.
Marine regions farther from the coasts are, in contrast, relatively poor in nutrients. The production of biomass and amounts of plankton that sink to the bottom are thus low there. As a result, methane hydrates very rarely occur in the deep sea at large distances from the coasts.
The zone in which gas hydrates are stable in the sea floor is called the gas hydrate stability zone (GHSZ). This is the area in which temperatures and pressures necessary for the formation of methane hydrates prevail. Above the GHSZ the ambient pressures are too low for the methane and water to react with one another. Below the GHSZ it is too warm due to proximity to the Earth’s hot interior. With every kilometre closer to the Earth’s core the temperature in the crust increases by 30 to 40 degrees Celsius. The thickness and position of the GHSZ vary from one marine region to another. In some cases the GHSZ is only a few metres thick and lies directly below the sea floor. In others it can be up to 800 metres thick and comprise massive sediment deposits.
Estimating amounts of methane hydrate
Until now only a few methane hydrate deposits in the ocean have been thoroughly studied. Nevertheless, attempts have been made to calculate the globally available amounts of methane hydrates. These resulted in estimates of 500 to 55,000 gigatonnes of carbon.
Carbon makes up 75 per cent of the mass of the methane molecule and can thus be used as a reference value. In this way the deposits can be compared with other fossil resource deposits.
The large differences in these estimates are primarily due to the fact that researchers had to consider vari-ous influencing variables in their calculations and weighted these differently. For an accurate estimate of the worldwide methane hydrate reserves the scientists will have to, firstly, calculate as accurately as possible how much biomass was deposited in the sediments over millions of years that then became available for meth-anogenesis. Secondly, they have to assess how much methane has been able to eventually penetrate into the GHSZ. The following are among the aspects that need to be considered:- climatic changes that have influenced the production of plankton and biomass through various epochs in the geological past;
- the activity of aerobic microorganisms and sulphate reducers that consume large amounts of the biomass in the upper layers of sediment;
- changes in the coastlines due to rising and falling sea levels during the glacial and interglacial periods. At certain times when marine regions were exposed there was no sedimentation at all. During other periods the sedimentation rates increased or decreased;
- the methane concentration in pore waters. Methane gas migrates upward through the pores, the water-filled spaces between sediment grains. The meth-ane concentration in the pore waters is greater or less depending on how much methane rises from deeper layers. Regardless of the prevailing pressures or temperatures, methane hydrate can only form when there is a sufficiently high concentration of methane in the pore water;
- plate tectonics: regions where one continental plate sinks beneath another, called subduction zones, are of particular interest. As the plate descends, the pore water is squeezed out of the sediments like a sponge. It rises, carrying its methane component with it. These processes continue today. When the methane reaches the GHSZ it can substantially contribute to the formation of methane hydrate. The challenge, then, is to accurately calculate the amounts of ascending water and methane in the subduction zones.
- 3.5 > Majestic stones: currents and waves have exposed ancient turbidites on the Point Loma Peninsula in California.

- More recent estimates of the worldwide amounts of gas hydrate, which attempt to consider all of these aspects, are on the order of 500 to 1500 gigatonnes of carbon. This is significantly less than the 55,000 gigatonnes that were postulated just a few years ago, but still de-cidedly more than all of the conventional reserves of natural gas, which today are projected at around 100 gigatonnes of carbon. In addition to the total estimates, detailed calculations of methane hydrate reserves in specific ocean regions are of interest to researchers. These would give clues as to where it is most worth-while to employ research ships for more targeted inves-tigations. Ship expeditions are extremely expensive. Energy companies and scientists thus have a primary interest in focusing on large deposits that could produce great amounts of methane in the future.
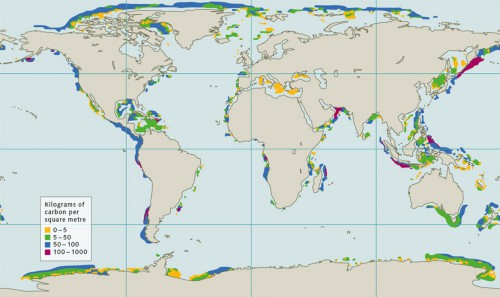
- 3.6 > Worldwide, methane hydrates occur primarily on the continental slopes. According to current estimates the largest deposits are located off Peru and the Arabian Peninsula. The figure only shows the biogenic gas hydrates. The amounts of thermogenic methane are not taken into account.
Promising layer-cake sediments
The amounts of methane, if any, that can be produced from the GHSZ primarily depend on the sediments in which the methane is located. There are various types of hydrate-bearing sediments that are distinguished by the proportions of smaller or larger particles: sands and sandstones, clays, and mixtures of these. Sands and sandstones have relatively large pore spaces, from which the methane can easily be retrieved. But there are only a few such sand bodies in the world that contain any methane hydrates at all. From compacted clay sediments, on the other hand, in which the particles are very dense, the methane hydrate cannot be recovered at all. Turbidites are widespread throughout the world. These are a combination of sand and clay sediments. In the layer-cake-like turbidite sediments, the sand and clay layers alternate. Over time, turbidites have formed primarily by mass slumps on the continental slopes. When too much sediment has been deposited a landslide begins on the slope. At the foot of the slope the sediments slide over one another in layers. Some of the turbidite layers are only a few centimetres thick. Occasionally, however, the individual layers can have thicknesses up to 10 metres. The feasibility of prod-ucing methane from hydrates in turbidites has been studied in recent months in test wells off Japan.