
The oceans – the largest CO2-reservoir
The mutability of carbon
Carbon is the element of life. The human body structure is based on it, and other animal and plant biomass such as leaves and wood consist predominantly of carbon (C). Plants on land and algae in the ocean assimilate it in the form of carbon dioxide (CO2) from the atmosphere or water, and transform it through photosynthesis into energy-rich molecules such as sugars and starches. Carbon constantly changes its state through the metabolism of organisms and by natural chemical processes. Carbon can be stored in and exchanges between particulate and dissolved inorganic and organic forms and exchanged with the the atmosphere as CO2. The oceans store much more carbon than the atmosphere and the terrestrial biosphere (plants and animals). Even more carbon, however, is stored in the lithosphere, i.e. the rocks on the planet, including limestones (calcium carbonate, CaCO3).
- The three most important repositories within the context of anthropogenic climate change – atmosphere, terrestrial biosphere and ocean – are constantly exchanging carbon. This process can occur over time spans of up to centuries, which at first glance appears quite slow. But considering that carbon remains bound up in the rocks of the Earth’s crust for millions of years, then the exchange between the atmosphere, terrestrial biosphere and ocean carbon reservoirs could actually be described as relatively rapid. Today scientists can estimate fairly accurately how much carbon is stored in the individual reservoirs. The ocean, with around 38,000 gigatons (Gt) of carbon (1 gigaton = 1 billion tons), contains 16 times as much carbon as the terrestrial biosphere, that is all plant and the underlying soils on our planet, and around 60 times as much as the pre-industrial atmosphere, i.e., at a time before people began to drastically alter the atmospheric CO2 content by the increased burning of coal, oil and gas. At that time the carbon content of the atmosphere was only around 600 gigatons of carbon. The ocean is therefore the greatest of the carbon reservoirs, and essentially determines the atmospheric CO2 content. The carbon, however, requires centuries to penetrate into the deep ocean, because the mixing of the oceans is a rather slow (Chapter 1). Consequently, changes in atmospheric carbon content that are induced by the oceans also occur over a time frame of centuries. In geological time that is quite fast, but from a human perspective it is too slow to extensively buffer climate change.
- With respect to climate change, the greenhouse gas CO2 is of primary interest in the global carbon cycle. Today, we know that the CO2 concentration in the atmosphere changed only slightly during the 12,000 years between the last ice age and the onset of the industrial revolution at the beginning of the 19th century. This relatively stable CO2 concentration suggests that the pre-industrial carbon cycle was largely in equilibrium with the atmosphere. It is assumed that, in this pre-industrial equilibrium state, the ocean released around 0.6 gigatons of carbon per year to the atmosphere. This is a result of the input of carbon from land plants carried by rivers to the ocean and, after decomposition by bacteria, released into the atmosphere as CO2, as well as from inorganic carbon from the weathering of continental rocks such as limestones. This transport presumably still occurs today at rates essentially unchanged. Since the beginning of the industrial age, increasing amounts of additional carbon have entered the atmosphere annually in the form of carbon dioxide.
- 2.1 > The carbon cycle in the 1990s with the sizes of the various reservoirs (in gigatons of carbon, Gt C), as well as the annual fluxes between these. Pre-industrial natural fluxes are shown in black, anthropogenic changes in red. The loss of 140 Gt C in the terrestrial biosphere reflects the cumulative CO2 emissions from land-use change (primarily slash and burn agriculture in the tropical rainforests), and is added to the 244 Gt C emitted by the burning of fossil fuels. The terrestrial sink for anthropogenic CO2 of 101 Gt C is not directly verifiable, but is derived from the difference between cumulative emissions (244 + 140 = 384 Gt C) and the combination of atmospheric increase (165 Gt C) and oceanic sinks (100 + 18 = 118 Gt C).
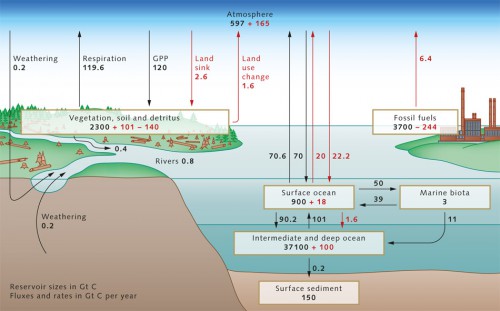
- The causes for this, in addition to the burning of fossil fuels (about 6.4 Gt C per year in the 1990s and more than 8 Gt C since 2006), include changes in land-use practices such as intensive slash and burn agriculture in the tropical rainforests (1.6 Gt C annually). From the early 19th to the end of the 20th century, humankind released around 400 Gt C in the form of carbon dioxide. This has created a serious imbalance in today’s carbon cycle. The additional input of carbon produces offsets between the carbon reservoirs, which lead to differences in the flux between reservoirs when compared to preindustrial times. In addition to the atmosphere, the oceans and presumably also land plants permanently absorb a portion of this anthropogenic CO2 (produced by human activity).
The ocean as a sink for anthropogenic CO2
As soon as CO2 migrates from the atmosphere into the water, it can react chemically with water molecules to form carbonic acid, which causes a shift in the concentrations of the hydrogen carbonate (HCO3-) and carbonate (CO32-) ions, which are derived from the carbonic acid. Because carbon dioxide is thus immediately processed in the sea, the CO2 capacity of the oceans is ten times higher than that of freshwater, and they therefore can absorb large quantities of it. Scientists refer to this kind of assimilation of CO2 as a sink. The ocean absorbs human-made atmospheric CO2, and this special property of seawater is primarily attributable to carbonation, which, at 10 per cent, represents a significant proportion of the dissolved inorganic carbon in the ocean. In the ocean, the carbon dissolved in the form of CO2, bicarbonate and carbonate is referred to as inorganic carbon. When a new carbon equilibrium between the atmosphere and the world ocean is re-established in the future, then the oceanic reservoir will have assimilated around 80 per cent of the anthropogenic CO2 from the atmosphere, primarily due to the reaction with carbonate. The buffering effect of deep-sea calcium carbonate sediments is also important. These ancient carbonates neutralize large amounts of CO2 by reacting with it, and dissolving to some extent. Thanks to these processes, the oceans could ultimately absorb around 95 per cent of the anthropogenic emissions. Because of the slow mixing of the ocean, however, it would take centuries before equilibrium is established. The very gradual buffering of CO2 by the reaction with carbonate sediments might even take millennia. For today’s situation this means that a marked carbon disequilibrium between the ocean and atmosphere will continue to exist for the decades and centuries to come. The world ocean cannot absorb the greenhouse gas as rapidly as it is emitted into the atmosphere by humans. The absorptive capacity of the oceans through chemical processes in the water is directly dependent on the rate of mixing in the world ocean. The current oceanic uptake of CO2 thus lags significantly behind its chemical capacity as the present-day CO2 emissions occur much faster than they can be processed by the ocean.
- 2.3 > Cement plants like this one in Amsterdam are, second to the burning of fossil fuels, among the most significant global sources of anthropogenic carbon dioxide. The potential for reducing CO2 output is accordingly large in these industrial areas.

Measuring exchange between the atmosphere and ocean
For dependable climate predictions it is extremely important to determine exactly how much CO2 is absorbed by the ocean sink. Researchers have therefore developed a variety of independent methods to quantify the present role of the ocean in the anthropogenically impacted carbon cycle. These have greatly contributed to the present-day understanding of the interrelationships. Two procedures in particular have played an important role:
- The first method (atmosphere-ocean flux) is based on the measurement of CO2 partial-pressure differences between the ocean surface and the atmosphere. Partial pressure is the amount of pressure that a particular gas such as CO2 within a gas mixture (the atmosphere) contributes to the total pressure. Partial pressure is thus also one possibility for quantitatively describing the composition of the atmosphere. If more of this gas is present, its partial pressure is higher. If two bodies, such as the atmosphere and the near-surface layers of the ocean, are in contact with each other, then a gas exchange between them can occur. In the case of a partial-pressure difference between the two media, there is a net exchange of CO2. The gas flows from the body with the higher partial pressure into that of lower pressure. This net gas exchange can be calculated when the global distribution of the CO2 partial-pressure difference is known. Considering the size of the world ocean this requires an enormous measurement effort. The worldwide fleet of research vessels is not nearly large enough for this task. A significant number of merchant vessels were therefore outfitted with measurement instruments that automatically carry out CO2 measurements and store the data during their voyages or even transmit them daily via satellite. This “Voluntary Observing Ship” project (VOS) has been developed and expanded over the last two decades and employs dozens of ships worldwide. It is fundamentally very difficult to adequately record the CO2 exchange in the world ocean, because it is constantly changing through space and time. Thanks to the existing VOS network, however, it has been possible to obtain measurements to provide an initial important basis. The database, covering over three decades, is sufficient to calculate the average annual gas exchange over the total surface of the oceans with some confidence. It is given as average annual CO2 flux density (expressed in mol C/m2/year), that is the net flux of CO2 per square meter of ocean surface per year, which can be integrated to yield the total annual CO2 uptake of the world ocean.
- Our present picture is based on around three million measurements that were collected and calculated for the CO2 net flux. The data were recorded between 1970 and 2007, and most of the values from the past decade were obtained through the VOS programme. Regions that are important for world climate such as the subpolar North Atlantic and the subpolar Pacific have been reasonably well covered. For other ocean regions, on the other hand, there are still only limited numbers of measurements. For these undersampled regions, the database is presently insufficient for a precise calculation. Still, scientists have been able to use the available data to fairly well quantify the oceanic CO2 sink. For the reference year 2000 the sink accounts for 1.4 Gt C.
- This value represents the net balance of the natural carbon flux out of the ocean into the atmosphere and, conversely, the transport of anthropogenic carbon from the atmosphere into the ocean. Now, as before, the annual natural pre-industrial amount of 0.6 Gt C is flowing out of the ocean. Conversely, around 2.0 Gt C of anthropogenic carbon is entering the ocean every year, leading to the observed balance uptake of 1.4 Gt C per year. Because of the still rather limited amount of data, this method has had to be restricted so far to the climatological CO2 flux, i.e., a long-term average over the entire observation period. Only now are studies beginning to approach the possibility of looking at interannual variability for this CO2 sink in especially well-covered regions. The North Atlantic is a first prominent example. Surprisingly, the data shows significant variations between individual years. Presumably, this is attributable to natural climate cycles such as the North Atlantic Oscillation, which have a considerable impact on the natural carbon cycle. Understanding such natural variability of the ocean is a prerequisite for reliable projections of future development and change of the oceanic sink for CO2.
- 2.4 > The world ocean takes up anthropogenic CO2 everywhere across its surface. The transport into the interior ocean, however, primarily takes place in the North Atlantic and in a belt between 30 and 50 degrees south latitude. The values indicate the total uptake from the beginning of the industrial revolution until the year 1994.

- The second method attempts, with the application of rather elaborate geochemical or statistical procedures, to calculate how much of the CO2 in the ocean is derived from natural sources and how much is from anthropogenic sources, although from a chemical aspect the two are basically identical, and cannot be clearly distinguished. Actually, several procedures are available today that allow this difficult differentiation, and they generally provide very consistent results. These methods differ, however, in detail, depending on the assumptions and approximations associated with a particular method. The most profound basis for estimating anthropogenic CO2 in the ocean is the global hydrographic GLODAP dataset (Global Ocean Data Analysis Project), which was obtained from 1990 to 1998 through large international research projects. This dataset:
- includes quality-controlled data on a suite of carbon and other relevant parameters;
- is based on analyses of more than 300,000 water samples;
- contains data that were collected on nearly 100 expeditions and almost 10,000 hydrographic stations in the ocean.
- 2.5 > In order to determine the effect of increasing atmospheric CO2 concentrations on the ocean, an international research team enriched seawater with CO2 in floating tanks off Spitsbergen, and studied the effects on organisms.

How climate change impacts the marine carbon cycle
The natural carbon cycle transports many billions of tons of carbon annually. In a physical sense, the carbon is spatially transported by ocean currents. Chemically, it changes from one state to another, for example, from inorganic to organic chemical compounds or vice versa. The foundation for this continuous transport and conversion is made up of a great number of biological, chemical and physical processes that constitute what is also known as carbon pumps. These processes are driven by climatic factors, or at least strongly influenced by them. One example is the metabolism of living organisms, which is stimulated by rising ambient temperatures. This temperature effect, however, is presumably less significant for the biomass producers (mostly single-celled algae) than for the biomass consumers (primarily the bacteria), which could cause a shift in the local organic carbon balance in some regions. Because many climatic interactions are still not well understood, it is difficult to predict how the carbon cycle and the carbon pumps will react to climate change. The first trends indicating change that have been detected throughout the world ocean are those of water temperature and salinity. In addition, a general decrease in the oxygen content of seawater has been observed, which can be attributed to biological and physical causes such as changes in current flow and higher temperatures. It is also possible that changes in the production and breakdown of biomass in the ocean play a role here.
- Changes in the carbon cycle are also becoming apparent in another way: The increasing accumulation of carbon dioxide in the sea leads to acidification of the oceans or, in chemical terms, a decline in the pH value. This could have a detrimental impact on marine organisms and ecosystems. Carbonate-secreting organisms are particularly susceptible to this because an acidifying environment is less favorable for carbonate production. Laboratory experiments have shown that acidification has a negative effect on the growth of corals and other organisms. The topic of ocean acidification is presently being studied in large research programmes worldwide. Conclusive results relating to the feedback effects between climate and acidification are thus not yet available. This is also the case for the impact of ocean warming. There are many indications for significant feedback effects here, but too little solid knowledge to draw any robust quantitative conclusions.
We will have to carry out focussed scientific studies to see what impact global change will have on the natural carbon cycle in the ocean. It would be naïve to assume that this is insignificant and irrelevant for the futureclimate of our planet. To the contrary, our limited knowledge of the relationships should motivate us to study the ocean even more intensely and to develop new methods of observation.
