
Climate change impacts on methane hydrates
How methane ends up in the ocean
People have been burning coal, oil and natural gas for more than a hundred years. Methane hydrates, on the other hand, have only recently come under controversial discussion as a potential future energy source from the oceana potential future energy source from the ocean. They represent a new and completely untapped reservoir of fossil fuel, because they contain, as their name suggests, immense amounts of methane, which is the main component of natural gas. Methane hydrates belong to a group of substances called clathrates – substances in which one molecule type forms a crystal-like cage structure and encloses another type of molecule. If the cage-forming molecule is water, it is called a hydrate. If the molecule trapped in the water cage is a gas, it is a gas hydrate, in this case methane hydrate.Further information on this topic is available here:
Methane hydrates can only form under very specific physical, chemical and geological conditions. High water pressures and low temperatures provide the best conditions for methane hydrate formationmethane hydrate formation. If the water is warm, however, the water pressure must be very high in order to press the water molecule into a clathrate cage. In this case, the hydrate only forms at great depths. If the water is very cold, the methane hydrates could conceivably form in shallower water depths, or even at atmospheric pressure. In the open ocean, where the average bottom-water temperatures are around 2 to 4 degrees Celsius, methane hydrates occur starting at depths of around 500 metres.Further information on this topic is available here:
- 2.16 > Methane hydrate looks like a piece of ice when it is brought up from the sea floor. This lump was retrieved during an expedition to the “hydrate ridge” off the coast of Oregon in the US.
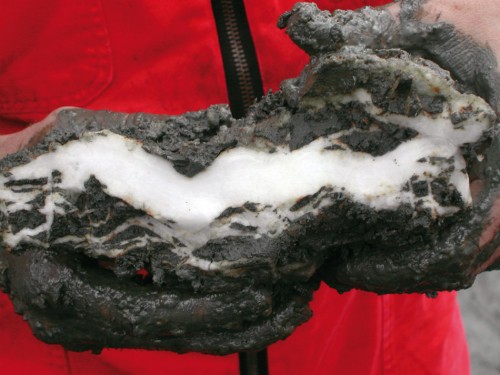
- Surprisingly, there is no methane hydrate in the deepest ocean regions, the areas with the highest pressures, because there is very little methane available here. The reason for this is because methane in the ocean is produced by microbes within the sea floor that break down organic matter that sinks down from the sunlit zone near the surface.
Organic matter is composed, for example, of the remains of dead algae and animals, as well as their excrements. In the deepest areas of the ocean, below around 2000 to 3000 metres, only a very small amount of organic remains reach the bottom because most of them are broken down by other organisms on their way down through the water column. As a rule of thumb, it can be said that only around 1 per cent of the organic material produced at the surface actually ends up in the deep sea. The deeper the sea floor is, the less organic matter settles on the bottom. Methane hydrates therefore primarily occur on the continental slopes, those areas where the continental plates meet the deep-sea regions. Here there is sufficient organic matter accumulating on the bottom and the combination of temperature and pressure is favourable. In very cold regions like the Arctic, methane hydrates even occur on the shallow continental shelf (less than 200 metres of water depth) or on the land in permafrost, the deep-frozen Arctic soil that does not even thaw in the summer.
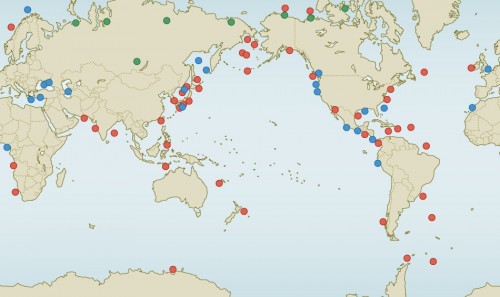
- 2.17 > Methane hydrate occurs in all of the oceans as well as on land. The green dots show occurrences in the northern permafrost regions. Occurrences identified by geophysical methods are indicated by red. The occurrences shown by blue dots were verified by direct sampling.
- It is estimated that there could be more potential fossil fuel contained in the methane hydrates than in the classic coal, oil and natural gas reserves. Depending on the mathematical model employed, present calculations of their abundance range between 100 and 530,000 gigatons of carbon. Values between 1000 and 5000 gigatons are most likely. That is around 100 to 500 times as much carbon as is released into the atmosphere annually by the burning of coal, oil and gas. Their possible future excavation would presumably only produce a portion of this as actual usable fuel, because many deposits are inaccessible, or the production would be too expensive or require too much effort. Even so, India, Japan, Korea and other countries are presently engaged in the development of mining techniques in order to be able to use methane hydrates as a source of energy in the future (Chapter 7).
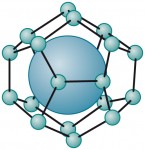 2.18 > In hydrates, the gas (large ball) is enclosed in a cage formed by water molecules. Scientists call this kind of molecular arrangement a clathrate.
2.18 > In hydrates, the gas (large ball) is enclosed in a cage formed by water molecules. Scientists call this kind of molecular arrangement a clathrate.Methane hydrates and global warming
Considering that methane hydrates only form under very specific conditions, it is conceivable that global warming, which as a matter of fact includes warming of the oceans, could affect the stability of gas hydrates. There are indications in the history of the Earth suggesting that climatic changes in the past could have led to the destabilization of methane hydrates and thus to the release of methane. These indications – including measurements of the methane content in ice cores, for instance – are still controversial. Yet be this as it may, the issue is highly topical and is of particular interest to scientists concerned with predicting the possible impacts of a temperature increase on the present deposits of methane hydrate.
Methane is a potent greenhouse gas, around 20 times more effective per molecule than carbon dioxide. An increased release from the ocean into the atmosphere could further intensify the greenhouse effect. Investigations of methane hydrates stability in dependance of temperature fluctuations, as well as of methane behaviour after it is released, are therefore urgently needed.

2.19 > Gas hydrates occur when sufficient methane is produced by organic matter degradation in the sea floor under low temperature and high pressure conditions. These conditions occur predominantly on the continental margins. The warmer the water, the larger the water depths must be to form the hydrate. Deep inside he sea floor, however, the temperature is too high for the formation of methane hydrates because of the Earth’s internal heat.
Oxidation Many bacteria use methane to provide energy for their metabolism. They take up methane and transform it chemically. In this process the methane releases electrons and is thus oxidized. Some bacteria break the methane down with the help of oxygen. This is called aerobic oxidation. Other bacteria do not need oxygen. This kind of oxidation is called anaerobic.
- Various methods are employed to predict the future development. These include, in particular, mathematic modelling. Computer models first calculate the hypothetical amount of methane hydrates in the sea floor using background data (organic content, pressure, temperature). Then the computer simulates the warming of the seawater, for instance, by 3 or 5 degrees Celsius per 100 years. In this way it is possible to determine how the methane hydrate will behave in different regions. Calculations of methane hydrate deposits can than be coupled with complex mathematical climate and ocean models. With these computer models we get a broad idea of how strongly the methane hydrates would break down under the various scenarios of temperature increase. Today it is assumed that in the worst case, with a steady warming of the ocean of 3 degrees Celsius, around 85 per cent of the methane trapped in the sea floor could be released into the water column.
Other, more sensitive models predict that methane hydrates at great water depths are not threatened by warming. According to these models, only the methane hydrates that are located directly at the boundaries of the stability zones would be primarily affected. At these locations, a temperature increase of only 1 degree Celsius would be sufficient to release large amounts of methane from the hydrates. The methane hydrates in the open ocean at around 500 metres of water depth, and deposits in the shallow regions of the Arctic would mainly be affected.
In the course of the Earth’s warming, it is also expected that sea level will rise due to melting of the polar ice caps and glacial ice. This inevitably results in greater pressure at the sea floor. The increase in pressure, however, would not be sufficient to counteract the effect of increasing temperature to dissolve the methane hydrates. According to the latest calculations, a sea-level rise of ten metres could slow down the methane-hydrate dissolution caused by a warming of one degree Celsius only by a few decades.
A wide variety of mathematical models are used to predict the consequences of global warming. The results of the simulations are likewise very variable. It is therefore difficult to precisely evaluate the consequences of global warming for the gas hydrate deposits, not least of all because of the large differences in the calculations of the size of the present-day gas hydrate deposits. One major goal of the current gas hydrate research is to optimize these models by using ever more precise input parameters. In order to achieve this, further measurements, expeditions, drilling and analyses are essential.
What happens when methane hydrate melts?
Not all the methane that is released from unstable methane hydrates ends up in the atmosphere. The greatest portion is likely to be broken down during its rise through the sediments and in the water column. This decomposition is mediated by two biological processes:- anaerobic oxidation of methane by bacteria and archaea (formerly called archaebacteria) within the sea floor;
- aerobic oxidation of methane by bacteria in the water column.
- A prerequisite for this kind of degradation is that the methane molecules are dissolved in water. Methane can only be degraded by the bacteria in this form. If the methane is released rapidly from the hydrates, it could rise in the form of gas bubbles that are not accessible by microorganisms. The microbial methane filter would thus fail, at least in part, if the methane hydrates break down very rapidly and large quantities of methane are released at once.
There is also a problem at shallow water depths, where the methane bubbles cannot completely dissolve in the water over the short distance from the sea floor to the atmosphere. In order to better understand such processes and to be able to make predictions about the functions of the microbial filters, researchers are currently investigating natural methane sources on the sea floor, so-called cold seeps, which constantly release larger quantities of methane. These include near-surface gas hydrate deposits, mud volcanoes, and natural-gas seeps in shallow marine regions. These seeps are a kind of natural model where the behaviour of methane in the ocean can be studied. If we understand how nature reacts to these methane seeps at the sea floor, it will help us to estimate the consequences of larger methane releases from gas hydrates. The data obtained at the methane seeps should also help to improve the precision of mathematical methane hydrate simulations.
- 2.20 > Large quantities of methane hydrate are stored not only in the sea floor, but also on land, especially in the perpetually frozen permafrost ground of the Russian tundra, such as here in the Russian republic of Komi. Scientists are concerned that the permafrost soils could melt due to global warming and thus release the methane hydrates.

- The disappearance of methane hydratesdisappearance of methane hydratescould have fatal consequences. Gas hydrates act like a cement that fills the pores between the fine sediment particles and stabilizes the sea floor. If the methane hydrates decompose, the stability of the sea floor is reduced due to the missing cement and the possible generation of excess pore pressure. In the worst case, large parts of continental margins fail. The resulting submarine landslides might cause severe tsunamis.Further information on this topic is available here:
Massive mass movements occurred during the last ice age and the following deglaciation. The trigger was probably not always warming of the atmosphere, but also the opposite. Because large quantities of water were stored in the ice during the last ice age, sea level was around 120 metres lower than it is today. Especially in the shallow ocean regions, the water pressure was so low that massive amounts of methane hydrate could have been destabilized. Direct evidences for such slope failures caused by decomposing gas hydrates have not yet been found. There are, however, some indications suggesting a process in the past. Signs of seeping fluids are almost always found in the vicinity of slope failures. These slopes were possibly destabilized by gases released by decomposing gas hydrates and liquids. Researchers also, however, definitely see the possibility of a reverse relationship: it is conceivable that slope failures and the resulting reduction in pressure on underlying sediments caused the dissociation of methane hydrates at the continental margins, thereby releasing large amounts of free gas. The slumps would have been the cause rather than the result of gas escape. These uncertainties highlight the need for further research. It is, however, fairly certain that the disappearance of methane hydrates could lead to serious problems.
Methane emissions from the Arctic – a prime focus of future gas hydrate research
In the field of methane emission research today, the Arctic is one of the most important regions worldwide. It is believed that methane occurs there both in the form of gas hydrate in the sea and as free gas trapped in the deep-frozen permafrost. Methane deposits in permafrost and hydrates are considered to be very sensitive in the expansive shallow-shelf regions, because with the relatively low pressures it would only take a small temperature change to release large amounts of methane. In addition, new methane is continuously being produced because the Arctic regions are rich in organic material that is decomposed by microbes in the sediment. The activity of these microbes and thus the biological release rates of methane are also stimulated by increases in temperature. Hence methane emissions in the Arctic have multiple sources. International scientific consortia are now being established involving researchers from various disciplines – chemists, biologists, geologists, geophysicists, meteorologists – which are intensively addressing this problem. No one can yet say with certainty how the methane release in the Arctic will develop with global warming, either in the ocean or on the land. This research is still in its infancy.