
Methane hydrates
Breeding ground for methane hydrates: The sea floor
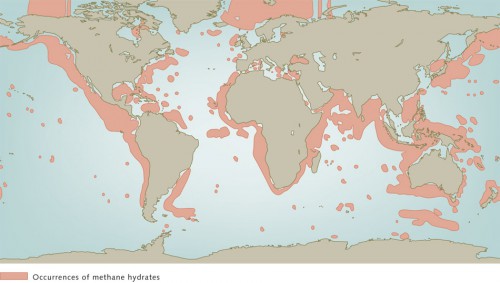
Methane hydrates are white, ice-like solids that consist of methane and water. The methane molecules are enclosed in microscopic cages composed of water molecules. Methane gas is primarily formed by microorganisms that live in the deep sediment layers and slowly convert organic substances to methane. These organic materials are the remains of plankton that lived in the ocean long ago, sank to the ocean floor, and were finally incorporated into the sediments. Methane hydrates are only stable under pressures in excess of 35 bar and at low temperatures. The sea floor is thus an ideal location for their formation: the bottom waters of the oceans and the deep seabed are almost uniformly cold, with temperatures from 0 to 4 degrees Celsius. In addition, below a water depth of about 350 metres, the pressure is sufficient to stabilize the hydrates. But with increasing depth into the thick sediment layers on the sea floor the temperatures begin to rise again because of the proximity to the Earth’s interior. In sediment depths greater than about 1 kilometre the temperatures rise to over 30 degrees Celsius, so that no methane hydrates can be deposited. This, however, is where the methane formation is especially vigorous. First, small methane gas bubbles are produced deep within the sediment. These then rise and are transformed to methane hydrates in the cooler pore waters near the sea floor. So the methane is formed in the deep warm sediment horizons and is converted and consolidated as methane hydrate in the cold upper sediment layers. No methane hydrates are found in marginal seas and shelf areas because the pressure at the sea floor is not sufficient to stabilize the hydrates. At the bottom of the expansive ocean basins, on the other hand, where the pressure is great enough, scarcely any hydrates are found because there is insufficient organic matter embedded in the deep-sea sediments. The reason for this is that in the open sea the water is comparatively nutrient poor, so that little biomass is produced to sink to the sea floor. Methane hydrates therefore occur mainly near the continental margins at water depths between 350 and 5000 metres. For one reason, enough organic material is deposited in the sediments there, and for another, the temperature and pressure conditions are favourable for methane to be converted to methane hydrates.
Greenhouse gas formation
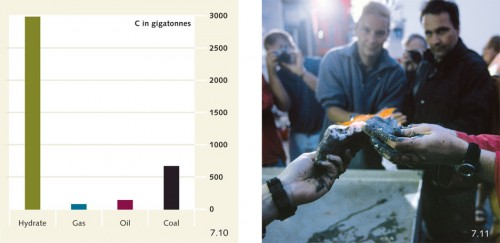
Vast amounts of methane hydrate are buried in sediment deposits on the continental slopes. The total global amount of methane carbon bound up in these hydrate deposits is in the order of 1000 to 5000 gigatonnes – i.e. about 100 to 500 times more carbon than is released annually into the atmosphere by the burning of fossil fuels (coal, oil and gas). At low temperatures the methane hydrates on the sea floor are stable, but if the water and the sea floor become warmer, then the hydrates can break down. Because microorganisms then oxidize the resulting methane gas to form the greenhouse gas carbon dioxide (CO2), methane hydrates have recently become a topic of intense discussion within the context of climate change. Methane, which itself acts as a strong greenhouse gas, does not escape directly out of the sea as methane because it is transformed into CO2. But the formation and release of carbon dioxide are considerable. An additional problem is that the oxygen in seawater is consumed through the formation of carbon dioxide (Chapter 2).
- 7.7 > It is known that methane hydrates are present throughout the world’s oceans, primarily on the continental margins. Estimates of the total amounts of the deposits, however, are still very inexact.
- In 2008 British and German researchers discovered gas seeps at a depth of 350 metres on the continental slope off Spitsbergen that are probably fed by melting hydrates. Long-term measurements of the water temperatures off Spitsbergen indicate that the bottom-water masses and thus also the slope sediments have significantly warmed in recent decades. Models also predict that the sea floor in Arctic areas will continue to heat up in the coming decades and centuries due to climate change. Scientists therefore fear that large quantities of methane hydrate will melt there in the future, releasing increased amounts of CO2 into the ocean and the atmosphere. The oxygen content of the seawater will decrease accordingly.
- Furthermore, the CO2 released not only contributes to further global warming, it also leads to acidification of the oceans (Chapter 2). Examples from the geological past support this scenario. Based on geological records it can be assumed that hydrates have broken down on a large scale numerous times in the Earth’s history, leading to extreme global warming and massive extinctions of organisms on the sea floor. Further investigations are necessary to determine the scale at which changes in the climate and oceans will accelerate in the future due to the release of methane gas at the sea floor.
A future energy source?
Although the immense methane hydrate occurrences represent a risk to the climate, they are also a potential energy source. The amount of natural gas bound up in the hydrates far exceeds the natural gas reserves in conventional deposits. Natural gas fed into the supply lines from conventional sources already consists of more than 95 per cent methane. Until now, mining hydrates in the ocean has been considered an expensive process. As resource prices rise, however, these reserves are becoming more attractive to the offshore industry. Many scientists estimate that mining the hydrates could be economically feasible at an oil price of about 50 to 60 US dollars per barrel. This implies that production would already be profitable today. Great efforts are presently being made to develop hydrate deposits, particularly in the territorial waters of Japan, China, India, South Korea and Taiwan.
7.8 > Methane hydrates occur worldwide. This ice-like block with a honeycomb structure was obtained from the sea floor during a research expedition off the coast of Oregon.
7.9 > In methane hydrates, the methane gas molecules are tightly enclosed in cages composed of water molecules. Increasing temperatures render the cages unstable, the gas escapes.
![Fig. 7.8: © http://de.wikipedia.org/wiki/Datei:Gashydrat_mit_Struktur.jpg [Stand: 05.10.2010], Fig. 7.9: © maribus (after IFM-GEOMAR) 7.8 > Methane hydrates occur worldwide. This ice-like block with a honeycomb structure was obtained from the sea floor during a research expedition off the coast of Oregon.<br />
© http://de.wikipedia.org/wiki/Datei:Gashydrat_mit_Struktur.jpg [Stand: 5.10.2010]
7.9 > In methane hydrates, the methane gas molecules are tightly enclosed in cages composed of water molecules. Increasing temperatures render the cages unstable, the gas escapes. © maribus (after IFM-GEOMAR)](https://worldoceanreview.com/en/files/2010/10/7_8-7_9-methane-hydrates-500x248.jpg)
Carbon dioxide storage in the ocean
At the same time, new technologies are being developed in Germany that may be useful for exploring and extracting the hydrates. The basic idea is very simple: the methane (CH4) is harvested from the hydrates by replacing it with CO2. Laboratory studies show that this is possible in theory because liquid carbon dioxide reacts spontaneously with methane hydrate. If this concept could become economically viable, it would be a win-win situation, because the gas exchange in the hydrates would be attractive both from a financial and a climate perspective.
- Natural gas is a relatively clean fossil fuel. CO2 emissions from gas-fired power plants are about 50 per cent lower than from conventional coal-fired plants. But even the emissions from modern gas-fired systems can be reduced considerably when CCS technology (carbon capture and storage) is installed. By this method the CO2 is isolated directly at the power plant and is stored in underground geological formations.
Another option would be to inject the CO2 into the marine methane hydrates; by this method, not only would methane gas be obtained, but the carbon dioxide would also be securely captured. Onshore, CO2 is stored as a supercritical fluid that is mobile and chemically very aggressive. Some experts are concerned that underground storage reservoirs could therefore start to leak after a time. If, instead, carbon dioxide is stored as a hydrate within the cold deep sea floor, it would be much safer, because CO2 hydrates are considerably more thermally stable than methane hydrates. Even warming of the sea floor would not destabilize them. But this approach also involves ecological risk. During hydrate excavation the methane could escape unchecked into the seawater.
- To eliminate this risk, only the very deep hydrate occurrences that are covered by fine-grained sediment layers at least 100 metres thick should be developed. This is the only way to enable the methane gas to be retrieved safely through a borehole without the possibility of its escaping into the environment. In addition, care must be taken to ensure that the formation pressure is not increased by more than 10 bar during retrieval of the gas, as the sediment layers could otherwise break open and allow large amounts of methane to escape.
7.10 > The amount of carbon stored in methane hydrates at the sea floor (C in gigatonnes) far exceeds that stored in oil, gas and coal.
7.11 > A group of marine scientists on the deck of a research vessel ignite methane gas released from a degrading hydrate block.
Is there a future for methane mining?
So far the necessary mining technology has only been tested under laboratory conditions. Many years of development work are still needed to be able to reliably evaluate the potentials and risks and to realize mining on an industrial scale. The extraction of natural gas from methane hydrates onshore was successfully tested for the first time in 2008 by Japanese and Canadian scientists. In northern regions, methane hydrates lie hundreds of metres beneath the permafrost sediments. It is cold enough and the pressure is sufficient for hydrates to form there too. In contrast to the deposits in the sea floor, however, these hydrate occurrences are easy to access and therefore suitable for production tests. The tests showed that it is possible to produce natural gas by breaking down methane hydrates through the introduction of heat or the release of pressure.
- The retrieval of methane by replacement with carbon dioxide will now be tested onshore. A Norwegian-American consortium is set to carry out a production test in Alaska. The first offshore attempts are then planned for 2012 to 2014 on the continental slope off Japan. How and when methane hydrates are finally mined in the future depends on the results of these field investigations. And of course the development of world market prices for natural gas and carbon dioxide emission rights are also pivotal to any decisions to begin offshore mining on a major scale.
